What are Endobronchial Valves?
Endobronchial valves are the first FDA-approved, minimally invasive devices available in the United States to treat patients with severe emphysema. These groundbreaking valves allow one lobe of the lung to deflate and release the trapped air. This lets healthier parts of the lung expand and take in more air.
 Patients treated with endobronchial valves have reported immediate relief, including:
Patients treated with endobronchial valves have reported immediate relief, including:
- Breathing easier with reduced shortness of breath
- Feeling more active and energetic
- Enjoying a significantly improved quality of life compared to untreated patients
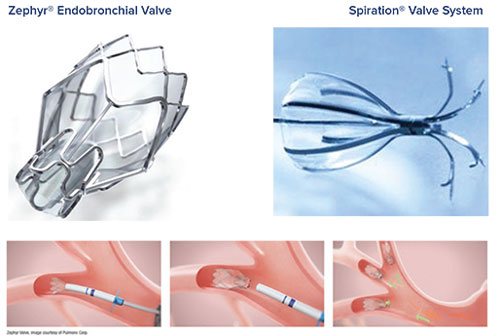
Inova uses two types of endobronchial valves: the Zephyr® Endobronchial Valve and the Spiration® Valve System. Our interventional pulmonology team will choose the best valve for each patient, based on their evaluation test results.
Who is Eligible for Endobronchial Valves?
Using specialized tests, our interventional pulmonology team determines in advance whether a patient is a good candidate for BLVR treatment with endobronchial valves. We conduct a diagnostic workup that may include pulmonary function testing, a CT scan, cardiac function tests and other tests to ensure the patient is a good candidate for treatment.
Patients must be referred to Inova for BLVR evaluation
If you’d like to discuss this treatment with your doctor, here is the eligibility criteria for BLVR evaluation:
- Smoke-free for at least four months
- Symptoms of breathlessness despite being on COPD medications
- Pulmonary function tests demonstrating severe obstruction (FEV1 < 45% predicted)
- Significant hyperinflation (e.g., RV > 150%, TLC > 100%)
- Six-minute walk distance of ≥ 100 meters
- Body mass index < 35kg/m2
- Clinical stability on ≤ 20mg prednisone daily

 Patients treated with endobronchial valves have reported immediate relief, including:
Patients treated with endobronchial valves have reported immediate relief, including: